Location of Double or Triple Bonds in Lipids Using Mass Spectrometry, Part I
DOI:
https://doi.org/10.54779/chl20230684Keywords:
double bond, fragmentation, mass spectrometry, lipids, ozonolysis, Paternò-Büchi, structural analysis, triple bondAbstract
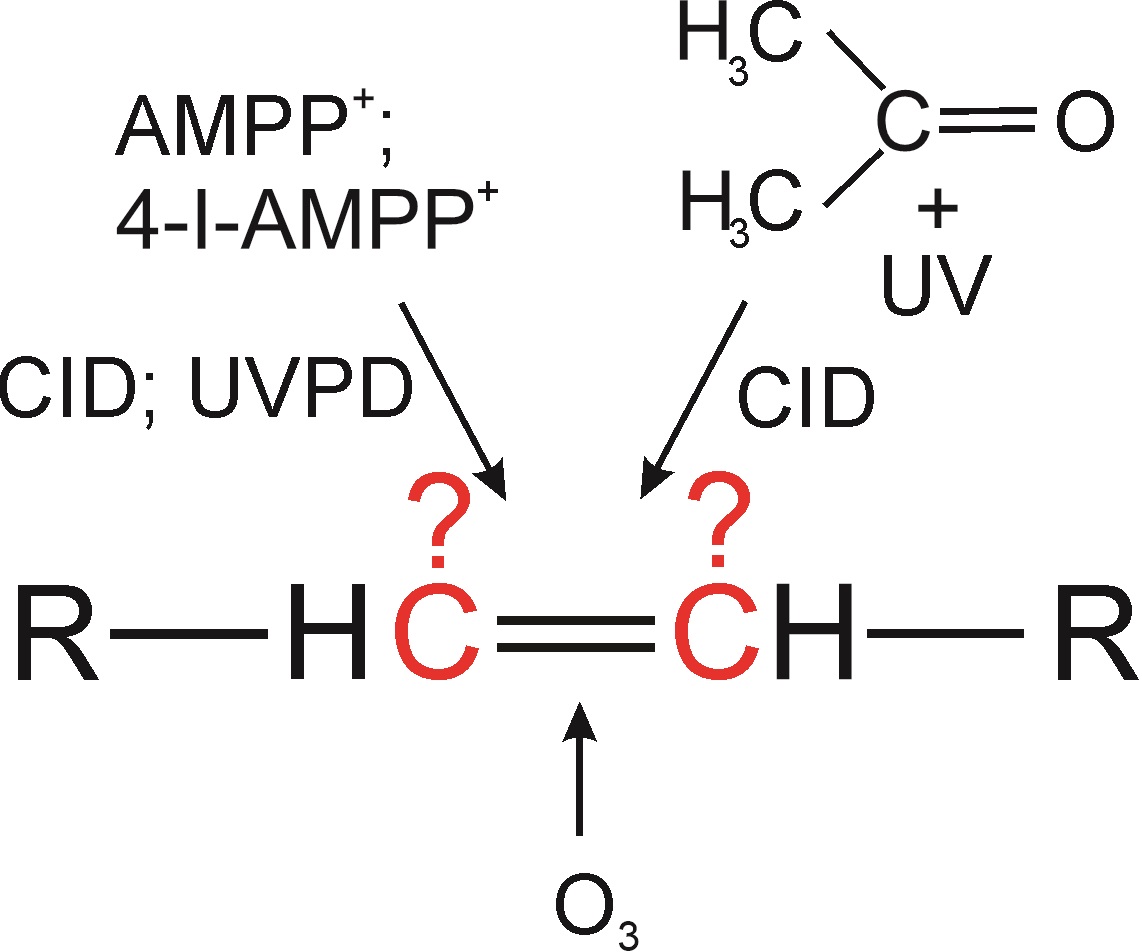
Structural analysis of lipids is one of the classical topics of organic mass spectrometry. In recent years, we have witnessed a rapid development of new methods that allow the characterization of lipid structures more accurately, more rapidly, and in more complex samples. These methods are mostly based on derivatization reactions or newly available fragmentation techniques. Basic mass spectrometric analysis of lipids, i.e., determination of lipid class, acyl chain length, and degree of unsaturation, is usually relatively easy but the detailed structural description is more challenging. In this two-part review article, we present advances in mass spectrometry for characterizing double and triple bonds in lipid acyl chains by LC-MS and direct ionization of liquid samples at atmospheric pressure. The first paper discusses charge-switch derivatizations, ozonolysis, and the Paternò-Büchi reaction.